Thermal explosion reaction coating on diamond surface of Cr/Al/B/diamond system
-
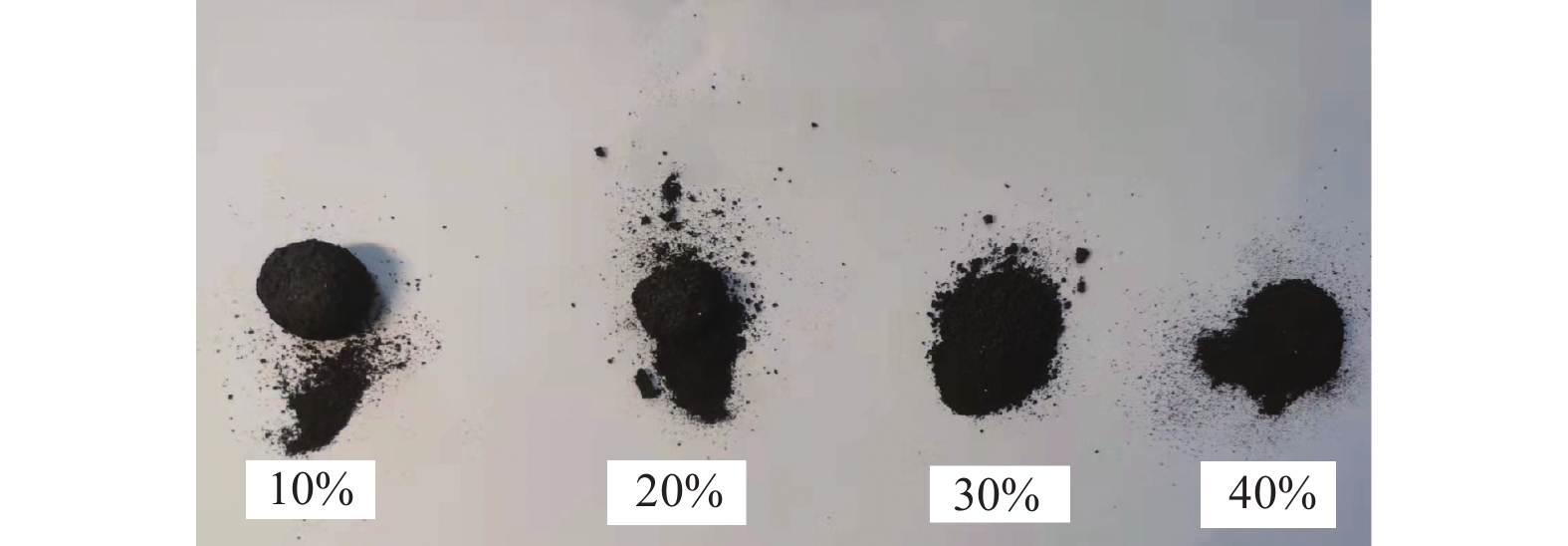
摘要: 采用Cr/Al/B/diamond粉体为原料,并添加少量Cr2O3或B2O3以诱发热爆反应。结果表明:在高纯Ar保护下,热爆反应后的试样粉末化严重,易将结合剂与金刚石颗粒分离。添加Cr2O3的原料体系发生热爆反应后,结合剂中的主相为Cr2AlB2,金刚石表面会形成含Cr3C2和Al的复合涂层,涂层的晶粒大小为0.5~7.0 µm。当金刚石质量分数为10%和20%时,试样中的金刚石颗粒表面涂覆良好,其起始和终止氧化温度都显著高于未涂覆金刚石的;而在金刚石质量分数较高时,其表面涂覆效果略差。添加B2O3的原料体系发生热爆反应后,金刚石表面的涂覆效果不佳,只有半数或以下数量的金刚石颗粒被涂覆。Abstract: Cr/Al/B/diamond powder was used as raw material, and a small amount of Cr2O3 or B2O3 was added to induce thermal explosion reaction. The results show that under the protection of high-purity Ar, the sample after thermal explosion reaction is seriously powdered, and it is easy to separate the binder from the diamond particles. After the thermal explosion reaction of the raw material system added with Cr2O3, the main phase in the binder is Cr2AlB2, and the composite coating containing Cr3C2 and Al will be formed on the diamond surface. The grain size of the composite coating is 0.5~7.0 µm. When the mass fractions of diamond are 10% and 20%, the surface of diamond particles in the samples are well coated, and the initial and the final oxidation temperatures of coated diamond are significantly higher than that of uncoated diamond. However, when the mass fraction of diamond is high, the coating effect on the diamond surface is slightly poor. After the thermal explosion reaction of the raw material system added with B2O3, the coating effect on the diamond surface is poor, and only half or less of the diamond particles are coated.
-
Key words:
- thermal explosion reaction /
- diamond particles /
- coating /
- oxidation resistance
-
表 1 Cr/Al/B结合剂中可能发生的化学反应焓变值
Table 1. Enthalpy changes of possible chemical reactions in Cr/Al/B binder
化学反应方程式 焓变值 △H / (kJ·mol−1) Cr + B = CrB −75.31 Cr + 2B = CrB2 −94.14 2Cr + 2B = Cr2B −38.53 2Cr + Al = Cr2Al −10.86 5Cr + 8Al = Cr5Al8 −17.67 Al + 2B = AlB2 −65.35 -
[1] 栗正新. 超硬磨具发展情况 [J]. 金刚石与磨料磨具工程,2021,41(2):1-3. doi: 10.3969/j.issn.1006-852X.2021.02.001LI Zhengxin. Development of superabrasives [J]. Diamond & Abrasives Engineering,2021,41(2):1-3. doi: 10.3969/j.issn.1006-852X.2021.02.001 [2] 万隆. 超硬材料磨具的研究和发展动态 [J]. 超硬材料工程,2009,21(1):40-42. doi: 10.3969/j.issn.1673-1433.2009.01.009WAN Long. Research and development trends of superhard material grinding tools [J]. Superhard Material Engineering,2009,21(1):40-42. doi: 10.3969/j.issn.1673-1433.2009.01.009 [3] HOU K H, WANG H T, SHEU H H, et al. Preparation and wear resistance of electrodeposited Ni–W/diamond composite coatings [J]. Applied Surface Science,2014,308(15):372-379. [4] OGIHARA H, HARA A, MIYAMOTO K, et al. Synthesis of super hard Ni–B/diamond composite coatings by wet processes [J]. Chemical Communications-Royal Society of Chemistry,2009,46(3):442-444. [5] HU G, YANG J, LIU Y. Deposition of tungsten-titanium carbides on surface of diamond by reactive PVD [J]. Transactions of Nonferrous Metals Society of China,1999,9(4):838-841. [6] SHOJIRO M, TAKANORI S, MASATOSHI M. Regression analysis of the effect of bias voltage on nano- and macro-tribological properties of diamond-like carbon films deposited by a filtered cathodic vacuum arc ion-plating method [J]. Journal of Nanomaterials, 2014,2014(1):1-13. [7] WALID M D, HEE S P, SOON H H. Fabrication of TiN/cBN and TiC/diamond coated particles by titanium deposition process [J]. Transactions of Nonferrous Metals Society of China,2014,24(11):3562-3570. doi: 10.1016/S1003-6326(14)63502-0 [8] JIAO X Y, CAI X P, NIU G, et al. Rapid reactive synthesis of TiAl3 intermetallics by thermal explosion and its oxidation resistance at high temperature [J]. Progress in Natural Science: Materials International,2019,29(4):447-452. doi: 10.1016/j.pnsc.2019.05.002 [9] LIU Y N, SUN Z, CAI X P, et al. Fabrication of porous FeAl-based intermetallics via thermal explosion [J]. Transactions of Nonferrous Metals Society of China. 2018, 28(6): 1141-1148, [10] ZHANG W X, LIANG B Y, LUO W, et al. Hybrid Ti2AlC bonded diamond composites prepared by a self-propagation sintering approach [J]. Journal of Wuhan University of Technology (Materials Science Edition),2019(1):82-85. [11] LIANG B Y, DAI Z, ZHANG Q, et al. Coating of diamond by thermal explosion reaction [J]. Diamond and Related Materials,2021,119:108572. doi: 10.1016/j.diamond.2021.108572 [12] LIANG B Y, GUO C P, LI C R, et al. Thermodynamic modeling of the Al–Cr system [J]. Journal of Alloys & Compounds,2016,460:314-319. -




 下载:
下载:











 邮件订阅
邮件订阅 RSS
RSS
