Thermal explosion reaction synthetic coating on diamond surface
-
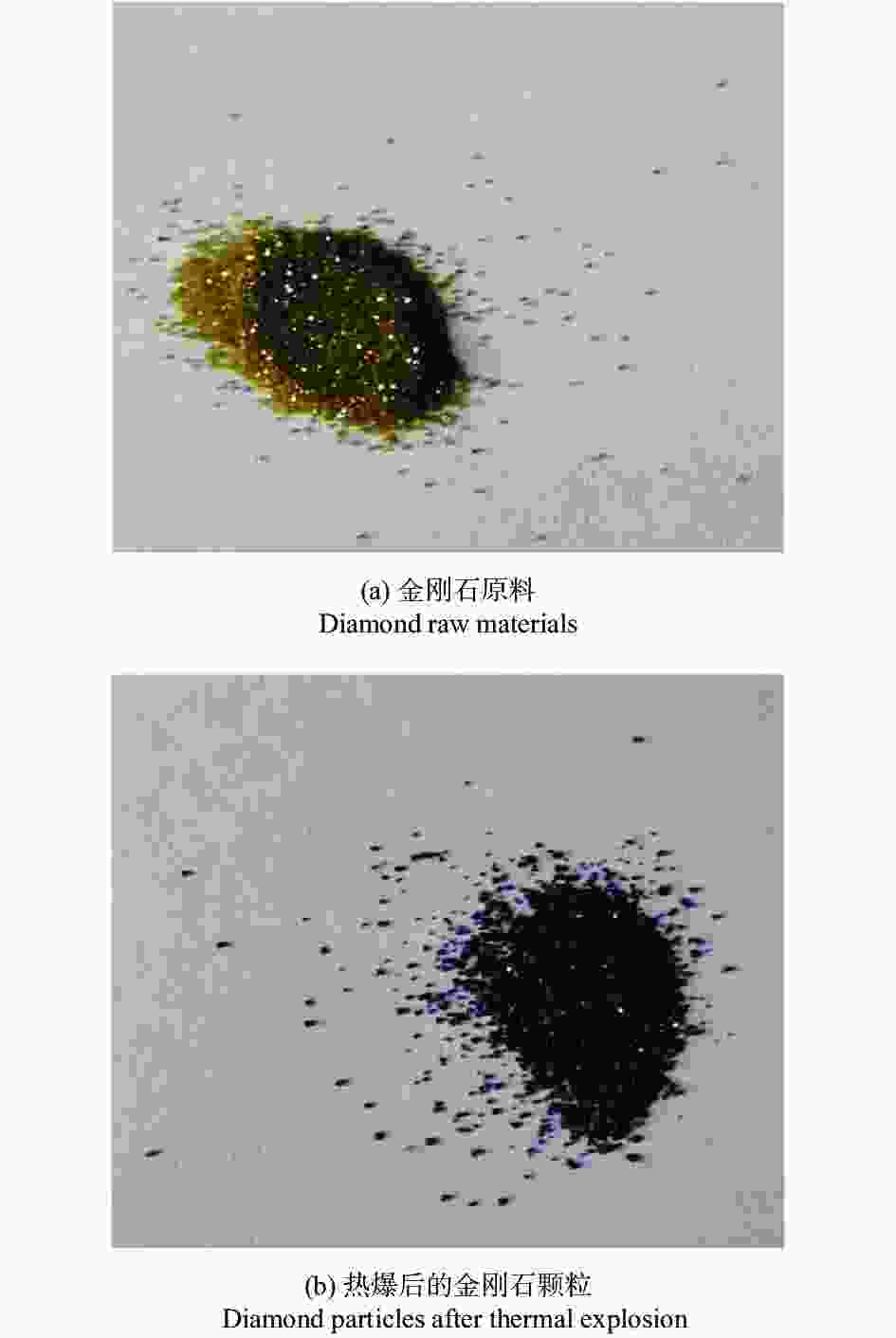
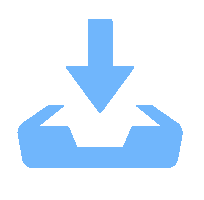
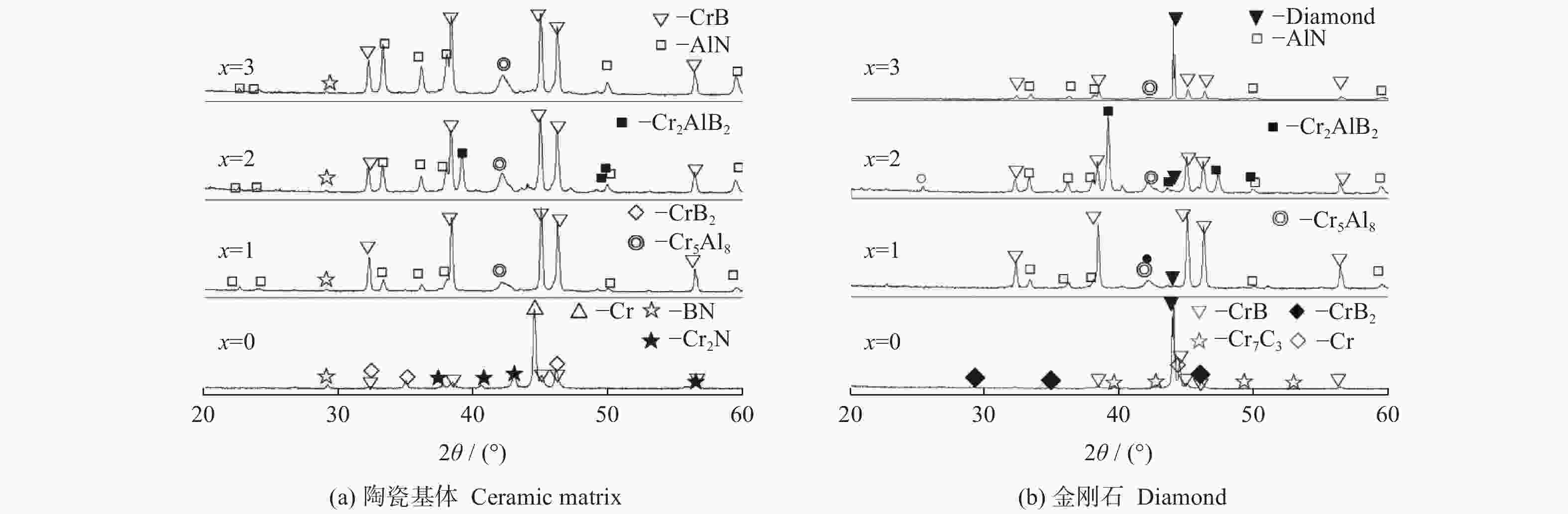
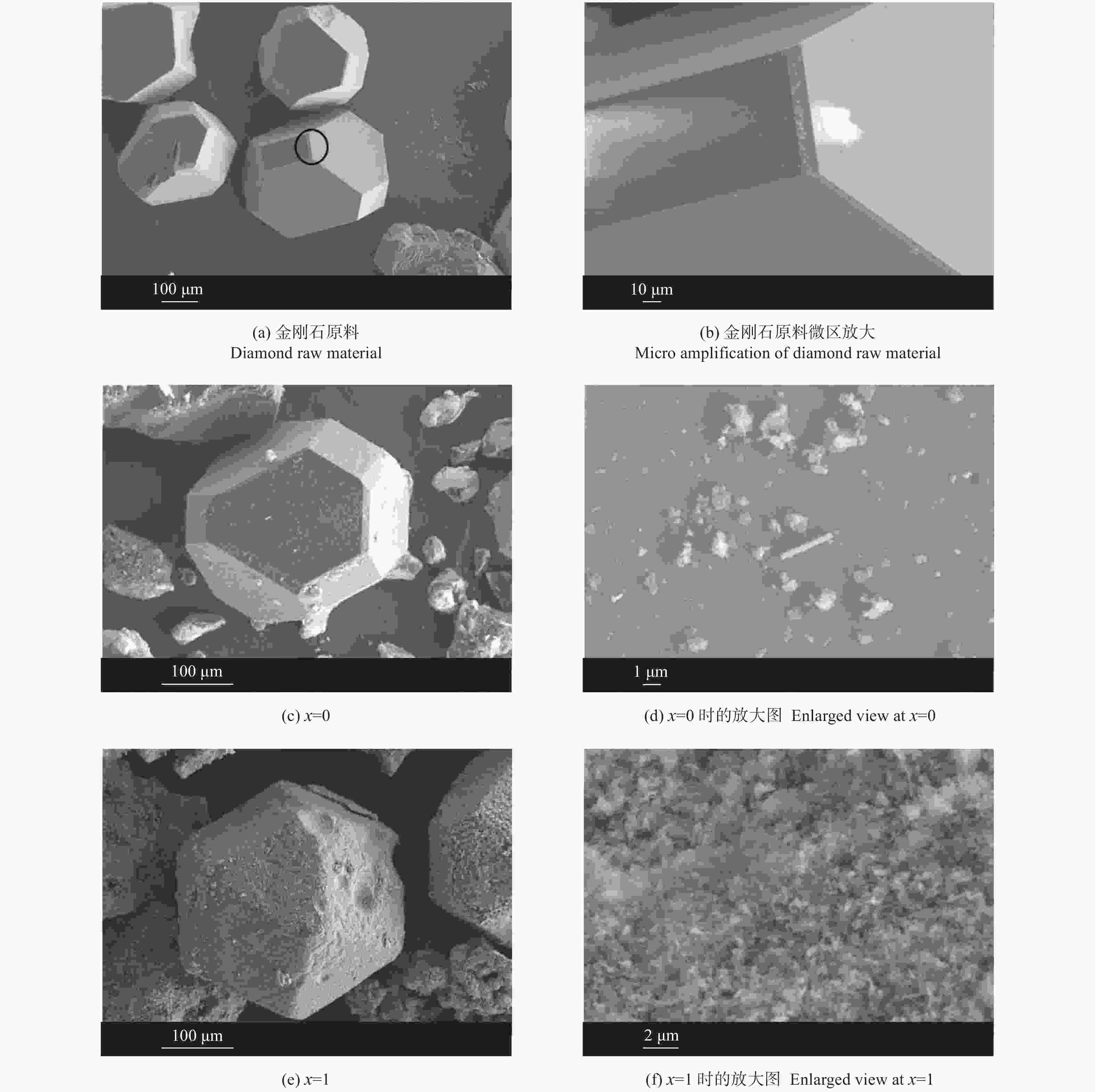
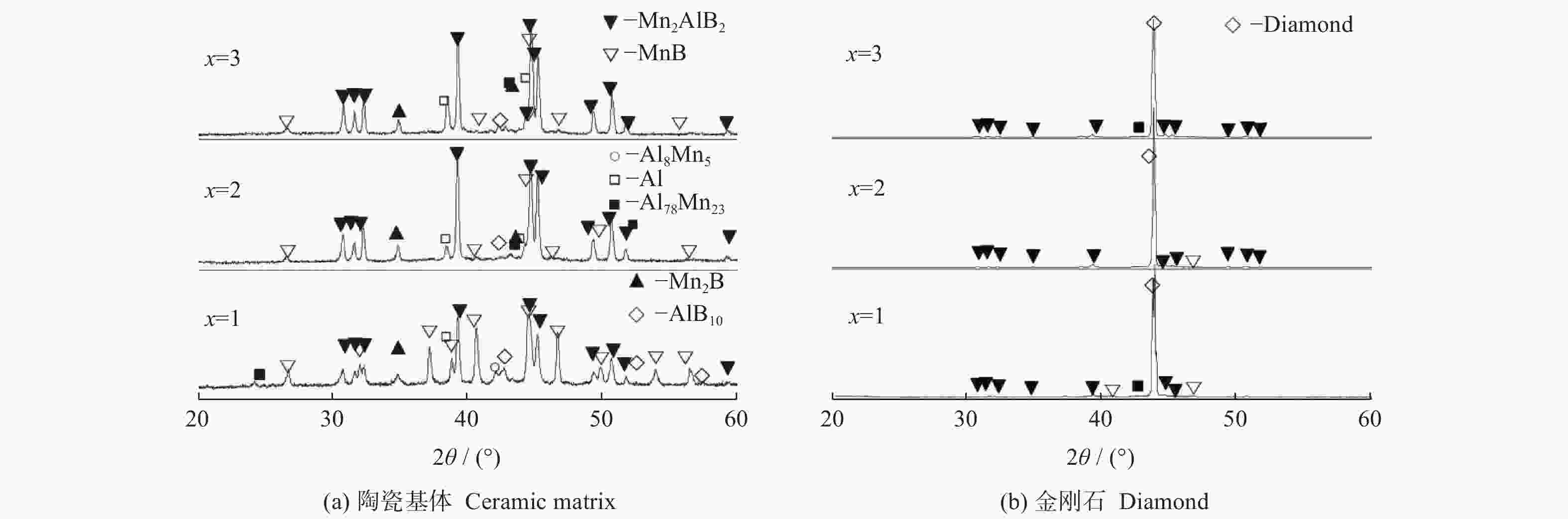
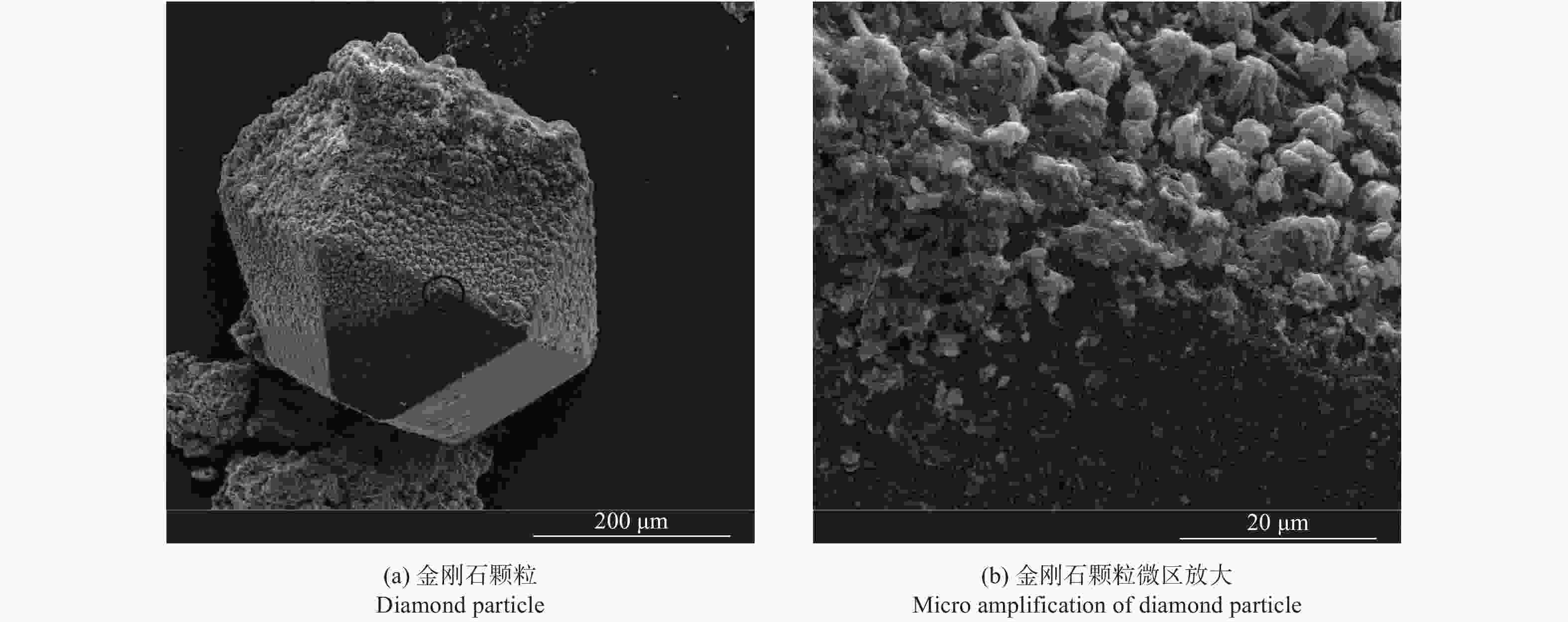
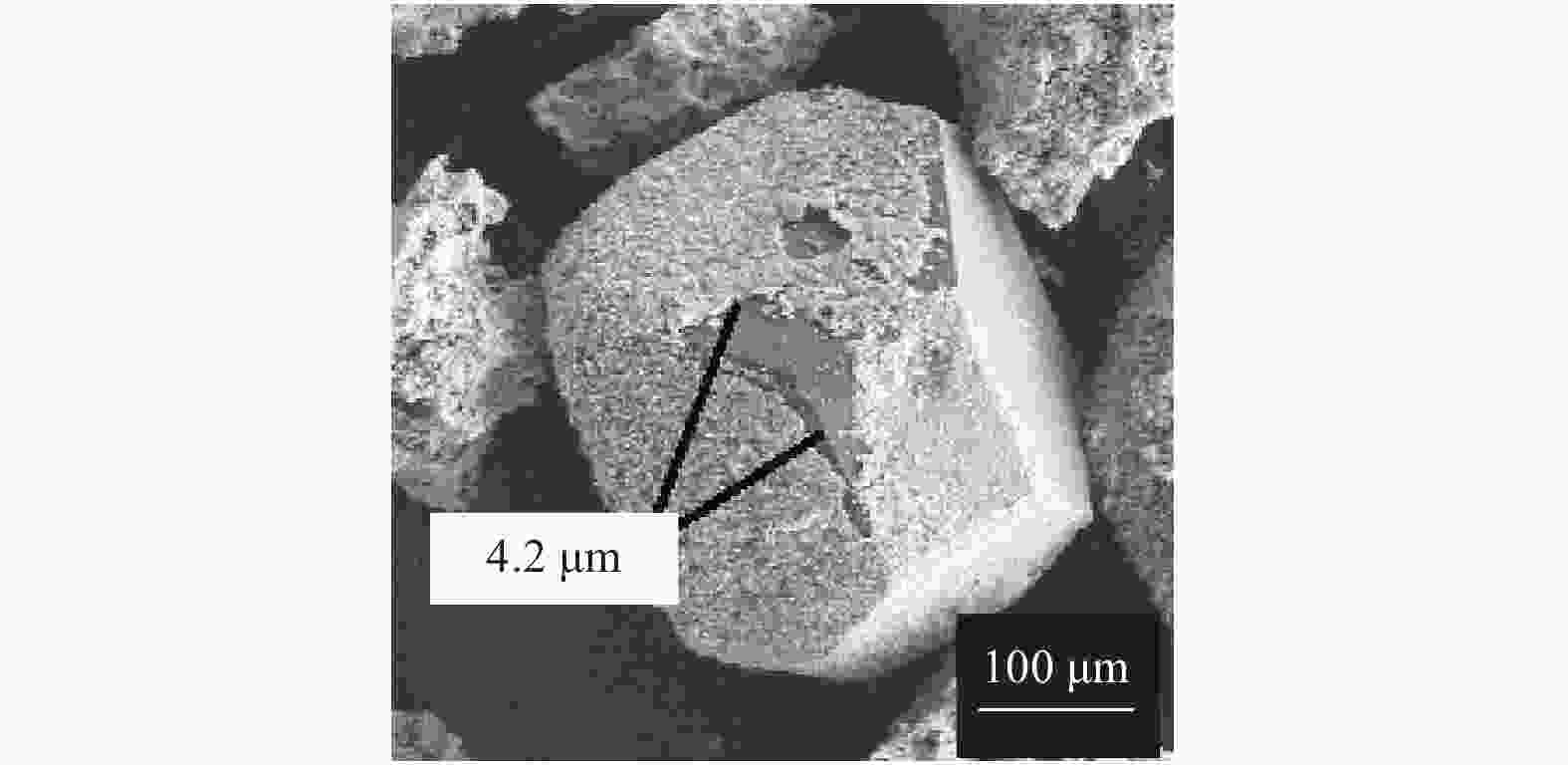
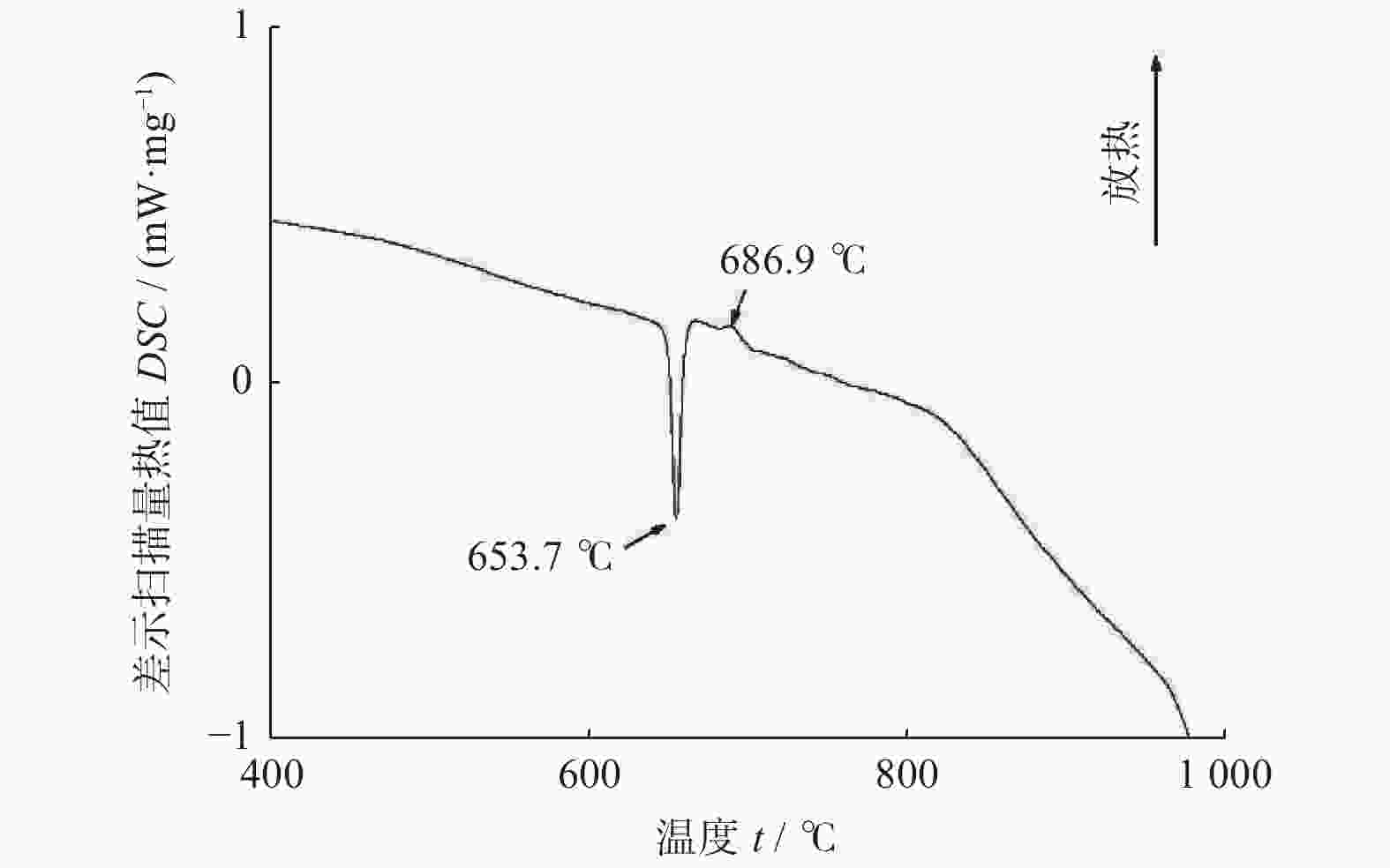
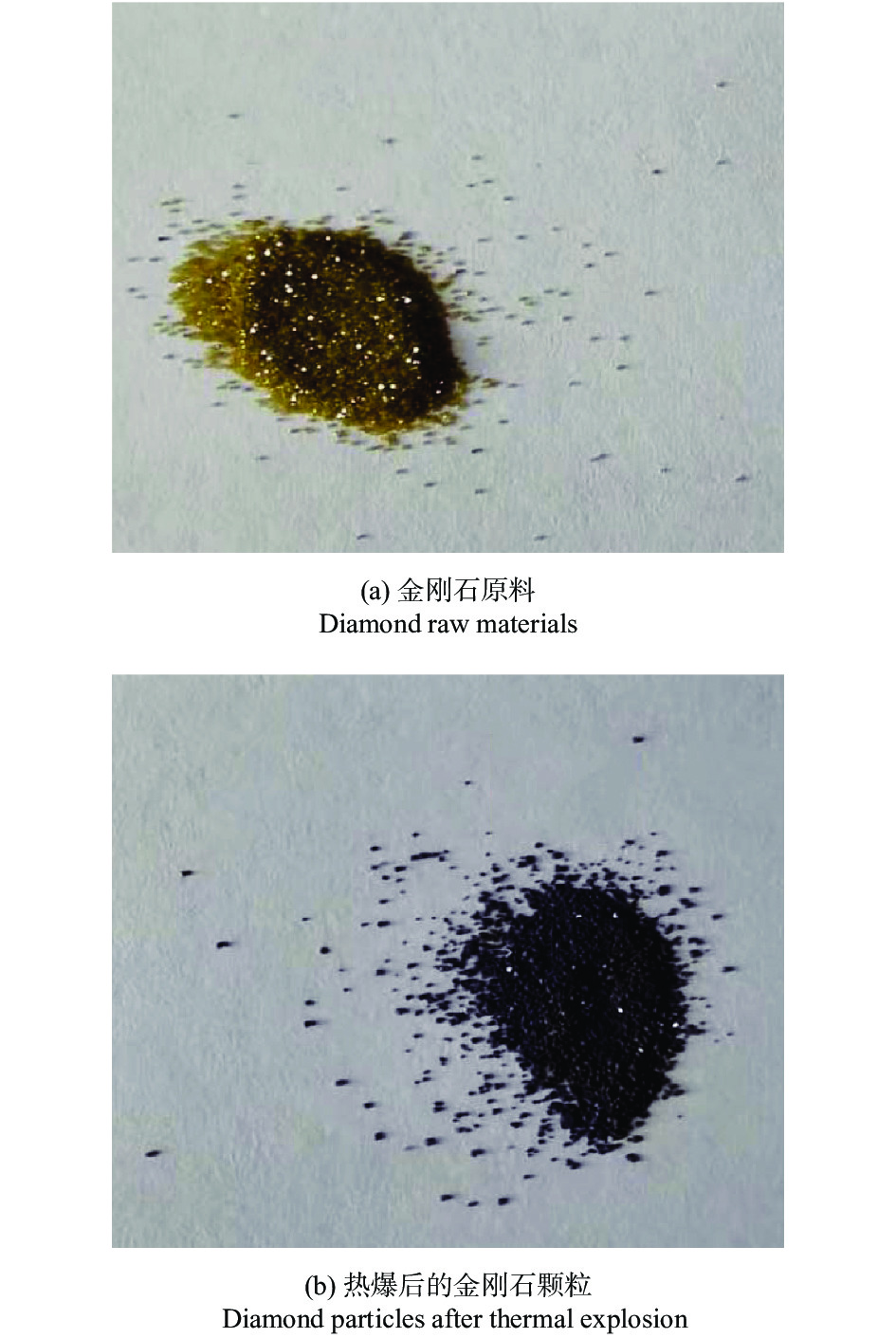
摘要: 以M(Mn或Cr)/Al/B/Diamond粉体为原料,通过热爆反应技术在金刚石表面生成多元复合涂层,并用X射线衍射仪、扫描电镜结合能谱仪研究2种原料体系及不同Al含量对陶瓷基体和涂层的物相组成和显微形貌的影响。结果表明:在N2的保护和引爆下,Cr/Al/B/Diamond粉体的热爆反应在金刚石表面形成CrB-AlN基多元复合涂层及Cr5Al8和Cr2AlB2等副产物;在Ar保护下,Mn/Al/B/Diamond粉体的热爆反应在金刚石表面形成Mn2AlB2基复合涂层。2种涂层对金刚石的包裹良好。2种热爆反应由于放热量较小,反应产物难以烧结成块体。制备出的疏松多孔块体易于粉碎和分离,从而可将陶瓷基体与金刚石颗粒分离。Abstract: Using M(Mn or Cr)/Al/B/diamond powders as raw materials, the multicomponent composite coatings were formed on the surface of diamond by thermal explosion reaction technology. The effects of two raw material systems and different Al contents on the phase compositions and the microstructures of ceramic matrix and coatings were studied by X-ray diffractometer, scanning electron microscope and energy dispersive spectrometer. The results show that under the protection and detonation of N2, the thermal explosion reaction of Cr/Al/B/diamond powder forms CrB-AlN based multicomponent composite coating and by-products such as Cr5Al8 and Cr2AlB2 on the surface of diamond. Under the protection of Ar gas, Mn2AlB2 based composite coating is formed on the surface of diamond by thermal explosion reaction of Mn/Al/B/diamond powder. The two coatings wrap diamond well. Due to the small heat release of the two thermal explosion reactions, the reaction products are difficult to sinter into blocks. The prepared loose porous blocks are easy to crush and separate.Therefore, the ceramic matrix can be separated from diamond particles.
-
Key words:
- coating /
- diamond /
- thermal explosion reaction
-
表 1 M(Cr或Mn)/Al/B体系中重要反应的标准摩尔焓变值
Table 1. Standard molar enthalpy changes of important reactions in M(Cr or Mn)/Al/B system
序号 反应 焓变值 ΔH / (kJ·mol−1) 文献 1 Cr + B = CrB −75.3 [18] 2 Cr + 2B = CrB2 −94.1 [18] 3 2Cr + Al = Cr2Al −12.2 [19] 4 8Cr + 5Al = Cr8Al5 −14.3 [19] 5 9Cr + 17Al = Cr9Al17 −15.7 [19] 6 Al + 2B = AlB2 −66.9 [18] 7 Mn + B = MnB −75.3 [18] 8 Mn + 2B = MnB2 −94.1 [18] 9 8Al + 5Mn = Al8Mn5 −25.8 [20] 10 6Al + Mn = Al6Mn −15.6 [20] 11 4Al + Mn = Al4Mn −21.2 [20] -
[1] 陈石林, 彭振斌, 陈启武. 聚晶金刚石复合体的研究进展 [J]. 矿冶工程,2004,24(2):85-89.CHEN Shilin, PENG Zhenbin, CHEN Qiwu. Advances in research work on polycrystalline diamond compacts [J]. Mining and Metallurgical Engineering,2004,24(2):85-89. [2] HOU K H, HAN T W, SHEU H H, et al. Preparation and wear resistance of electrodeposited Ni–W/diamond composite coatings [J]. Applied Surface Science,2014,308(15):372-379. [3] LIANG X, JIA C, CHU K, et al. Thermal conductivity and microstructure of Al/diamond composites with Ti-coated diamond particles consolidated by spark plasma sintering [J]. Journal of Composite Materials,2012,46(9):1127-1136. doi: 10.1177/0021998311413689 [4] LI R, FENG Y, WANG X, et al. The simultaneous deposition and growth mechanism of diamond-like carbon films on both surfaces of stainless steel substrate by electrodeposition [J]. Materials Review,2016,30(2):56-60. [5] LIN B, WANG X, ZHANG Y, et al. Interface characterization of a Cu-Ti-coated diamond system [J]. Surface and Coatings Technology,2015,278:163-170. doi: 10.1016/j.surfcoat.2015.08.006 [6] MIYAKE S, SHINDO T, MIYAKE M. Regression analysis of the effect of bias voltage on nano- and macrotribological properties of diamond-like carbon films deposited by a filtered cathodic vacuum arc ion-plating method [J]. Journal of Nanomaterials, 2014,2014(1):1-13. [7] WALID M D, HEE S P, SOON H H. Fabrication of TiN/cBN and TiC/diamond coated particles by titanium deposition process [J]. Transactions of Nonferrous Metals Society of China,2014,24(11):3562-3570. doi: 10.1016/S1003-6326(14)63502-0 [8] ROMMEL D, SCHERM F, KUTTNER C, et al. Laser cladding of diamond tools: Interfacial reactions of diamond and molten metal [J]. Surface and Coatings Technology,2016(291):62-69. [9] OKADA T, FUKUOKA K, ARATA Y, et al. Tungsten carbide coating on diamond particles in molten mixture of Na2CO3 and NaCl [J]. Diamond & Related Materials,2015,52:11-17. [10] MERZHANOVA G. Combustion processes that synthesize materials [J]. Journal of Material Process Technology,1996,56(1/2/3/4):222-241. doi: 10.1016/0924-0136(95)01837-9 [11] THIERS L, MUKASYAN A S, VARMA A. Thermal explosion in Ni-Al system: Influence of reaction medium microstructure [J]. Combustion and Flame,2002,131(1/2):198-209. doi: 10.1016/S0010-2180(02)00402-9 [12] JIAO X Y, CAI X P, NIU G, et al. Rapid reactive synthesis of TiAl3 intermetallics by thermal explosion and its oxidation resistance at high temperature [J]. Progress in Natural Science:Materials International,2019,29(4):447-452. doi: 10.1016/j.pnsc.2019.05.002 [13] LIU Y, SUN Z, CAI X, et al. Fabrication of porous FeAl-based intermetallics via thermal explosion [J]. Transactions of Nonferrous Metals Society of China,2018,28(6):1141-1148. doi: 10.1016/S1003-6326(18)64737-5 [14] ZHANG F, YUAN H, WANG C,et al. Microstructure of Ni-Al-Diamond composite fabricated by self propagating high temperature synthesis [J]. Key Engineering Materials,2005(291/292):531-534. [15] XU X, GUO P, ZUO X, et al. Understanding the effect of Al/Ti ratio on the tribocorrosion performance of Al/Ti co-doped diamond-like carbon films for marine applications [J]. Surface and Coatings Technology,2020,40:126347. doi: 10.1016/j.surfcoat.2020.126347 [16] LIANG B, WANG Z, WANG L, et al. Self-propagation high-temperature sintering of the Ti-Al-C-diamond/BN system [J]. International Journal of Materials Research,2014,105(4):417-420. doi: 10.3139/146.111039 [17] YEMBADI R, PANIGRAHI B B. Thermodynamic assessments and mechanically activated synthesis of ultrafine Cr2AlC MAX phase powders [J]. Advanced Powder Technology,2017,28(3):732-739. doi: 10.1016/j.apt.2016.11.020 [18] 叶大伦, 胡建华. 实用无机物热力学数据手册 [M]. 北京: 冶金工业出版社, 2002.YE Dalun, HU Jianhua. Practical inorganic thermodynamic data book [M]. Beijing: Metallurgical Industry Press, 2002. [19] CUI S, JUNG I, KIM J, et al. A coupled experimental and thermodynamic study of the Al-Cr and Al-Cr-Mg systems [J]. Journal of Alloys and Compounds,2017,698:1038-1057. doi: 10.1016/j.jallcom.2016.12.298 [20] LIU X, OHNUMA I, KAINUMA R, et al. Thermodynamic assessment of the aluminum-manganese (Al-Mn) binary phase diagram [J]. Journal of Phase Equilibria,1999,20(1):45-56. doi: 10.1361/105497199770335938 -



 下载:
下载:



 邮件订阅
邮件订阅 RSS
RSS
